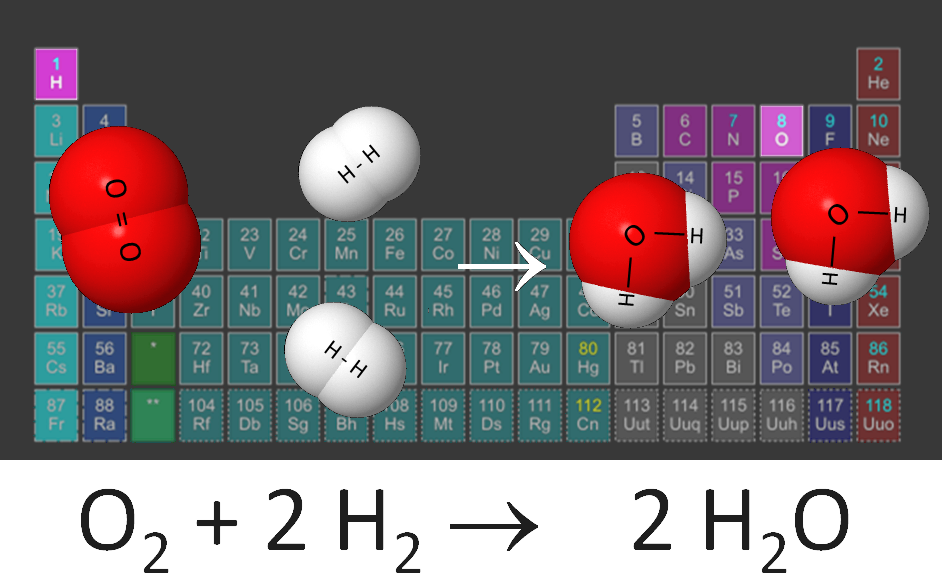
A molecule is a group of two or more atoms joined together. The atoms can be the same kind or different kinds . A compound is a substance that is made of two or more kinds of atoms joined together. An atom is made of electrons, protons, and neutrons. The mass of an atom is centered in its nucleus, where different numbers of protons and neutrons are combined, forming a variety of chemical elements. Water is a molecule made of two hydrogen atoms joined to an oxygen atom. This means that water is a molecule of a compound.
Elements Pure substances in which molecules are composed of only one kind of atoms are called elements. A pure substance is homogeneous in nature and has a definite set of properties. A compound is a pure substance made up of two or more elements combined chemically in a fixed proportion. Element Definition is “The basic idea of the finite element method is to divide the solution domain into a finite number of subdomains (elements). The number of protons they possess is determined by atoms. The atoms in an element all have the same number of protons, but electrons and neutrons can have different numbers.
Molecule vs Compound
An atom is a matter particle which characterises a chemical element. This is the smallest aspect of ordinary matter. It is composed of particles called protons, neutrons and subatomic particles called electrons. An element is a substance that is made entirely of one type of atom”. For example, the element sodium is made up of only sodium atoms. A compound is a pure substance consisting of chemically bound two or more distinct atoms to each other. Via chemical means, a substance may be dissolved. It may be broken down into smaller compounds, into modules or a mixture of the two.
Molecules consist of chemically bound several atoms. They can consist of one or more atom types. For repeating groups, certain molecules are lengthy. A compound is a molecule that contains more than one type of atom. Compounds are like baking a cake, kind of. It would not cook just correctly to mix the ingredients in the exact right proportions or the cake. A molecule definition is a particle made up of two or more atoms that are chemically bound together a given number is the number of atomic nuclei that make up a molecule.In a compound, if you change one molecule, then it becomes something totally new. If the oxygen atom is separated from water, you now have pure hydrogen.
Elements and Compounds
A compound is a pure substance that contains two or more elements. The elements are not just mixed together. Carbon, sulphur, oxygen, iron, copper and aluminium are typical examples. By symbols, components are depicted. Compounds are compounds that are composed of atoms of various elements that are joined by chemical bonds. Pure chemical entities present in nature are elements and compounds. The distinction between an element and a compound is that an element is a product made up of atoms of the same kind, while a compound is made up, in definite proportions, of various elements.
The central forms of chemistry are organic compounds, as important for understanding science as the definition of organisms if there is only one understanding of the biological sciences. They are elements, compounds, or mixtures: elements are only those that as ingredients do not include any other chemical substances.